Antibodies, Malaria and Mice: the Beginning of a Wonderful Friendship
18 Jun, 2007 12:49 pm
Malaria is a parasitic disease transmitted by female Anopheles mosquitoes. It is widespread in the tropical and subtropical regions of the world; however, 90% of all cases are in sub-Saharan Africa. Malaria, which now rivals HIV/AIDS as the world?s most deadly infection, infects 300-500 million people annually and kills a child every 30 seconds. The most serious forms of this disease in humans are caused by the parasite species Plasmodium falciparum and Plasmodium vivax.
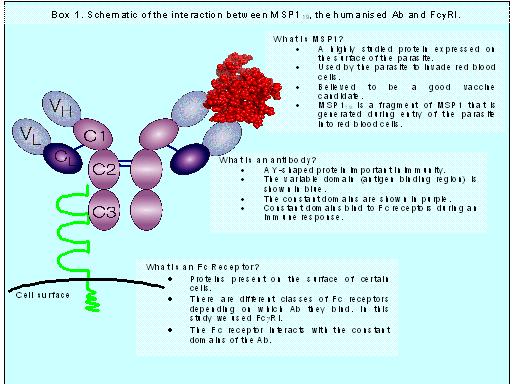
Antibodies (Abs) are molecules found in serum and are mainly produced in response to "foreign" molecules called antigens (see box 1). Abs interact with cells of the immune system through specific receptors, also known as Fc receptors.
The success of passive immunization (in which Abs from an immune donor are transferred to a naïve recipient) against malaria suggests that Ab-based therapies are potential alternatives. Abs play a crucial role in protective immunity to malaria, but their effectiveness can be enhanced by genetically engineered modifications. Abs or their fragments are the paradigm for high-affinity protein-based drugs and represent over 25% of all proteins undergoing clinical trials. Although at least 18 Abs have US Food and Drug Administration (FDA) approval, and more than 400 are currently in clinical trials, none of these Abs are in development for the treatment of malaria.
In our study, a phage library of Ab genes was made from adult Gambians immune to Plasmodium falciparum malaria. This library was then screened and the Abs with the highest affinity towards a parasite protein, called MSP119, were selected. The variable (antigen-specific) genes were then engrafted onto a human constant region, the backbone of the Ab that interacts with Fc receptors, creating a novel fully human Ab.
In order to explore the little-understood immunity involved in malaria, we created a transgenic in vivo mouse model that provides us with a way to assess Fc-dependent immunity that cannot be evaluated using in vitro assays. We infected the mice with P. bergei, a rodent malaria, which was transgenic for P. falciparum MSP119, the form of malaria that infects humans. The mice were also transgenic for one particular human Fc receptor (FcgRI) thus creating a model that mimics P. falciparum infection in humans.
Using this novel model, we could study the role of Abs and FcgRI in suppressing parasitemia. Firstly, we showed that Abs were necessary to cure the mice of malaria. Secondly, the disease was suppressed in the FcgRI transgenic mice but not in the non-transgenic equivalents. This indicated that the mere blocking of the MSP119 function is insufficient to bring about protection, and therefore demonstrated that the presence of the Fc region is crucial for the recruitment of FcgRI-mediated parasite clearance. This was further confirmed by reversing protection in mice using a monoclonal Ab that blocks the FcgRI receptorís binding site. In summary, our results show that our fully human Abs were functional and that they could suppress parasitemia via the human FcgRI.
Our findings have important implications regarding the development of effective vaccines for this devastating disease. Our model not only provides a test for the efficacy of our therapeutic Abs, but also brings us a little closer to understanding the underlying immune mechanisms involved in malaria. This is essential if Abs are to be developed successfully as therapeutic entities.
Reference:
McIntosh RS, Shi J, et al. (2007) The importance of human FcgRI in mediating protection to malaria. PLoS Pathog 3(5):e72.doi:10.1371/journal.ppat.0030072







The proposed article is highly interesting and doesn't need any revision to me. I suggest to accept it without any modification.
Yours sincerely,
Agn?s Aubouy