Role of the Rb tumor suppressor in regulation of stem cell proliferation
The first tumor suppressor gene was identified in the 1950s and was designated Retinoblastoma (RB) because of the tumors that formed on the retina of the eye when the gene was mutated (Vogel 1979). Although it is most renowned for its role in pediatric eye cancer, the RB gene and genes that operate in the RB pathway are frequently inactivated in most types of human cancer. The study of how RB functions normally and why it is deregulated in cancer has pioneered the way for research on other tumor suppressors. Ablation of the Rb gene in mice has revealed that, in addition to suppressing cancer, Rb plays a critical role in the development of embryos in utero (Clarke et al. 1992; Jacks et al. 1992; Lee et al. 1992; de Bruin et al. 2003; Wu et al. 2003). In a paper published in January 2007, we show that stem cells in the placenta require Rb for proper regulation of placental development (Wenzel et al. 2007). Loss of Rb function in placenta stem cells caused uncontrolled growth and architectural disruption of the placenta, leading to subsequent death of the fetus. We discuss the implications of these findings in the context of their relevance to human pregnancies, stem cell biology, and cancer.
Retinoblastoma is the most common primary malignant eye tumor of childhood and affects most patients prior to two years of age. There are two forms of retinoblastoma, one is hereditary and the other is nonhereditary. Patients with the hereditary form of retinoblastoma inherit a mutated RB gene from their father or mother. These patients typically develop tumors in both eyes and are predisposed later in life to cancers throughout the body, affecting organs such as bone, brain, and soft tissues. In contrast, nonhereditary retinoblastoma is caused by sporadic mutation of the RB gene in the cells of the retina, and these patients do not have an increased risk of developing other cancer types. Interestingly, two related genes, p107 and p130, have overlapping functions that can compensate for loss of RB in some tissues. Yet, loss of p107 or p130 alone does not cause cancer in humans as does loss of RB.
Study of the RB gene has in many ways pioneered a path to our current understanding of tumor suppressor genes. RB was the first tumor suppressor gene identified and evolutionarily related genes have been studied over the past 20 years in mouse (Rb), fly (rbf1), worm (lin-35), and most recently in yeast (WHI5). It is well known that the protein encoded by the RB gene (RB) plays a critical role in the control of cell division and regulates cellular processes such as proliferation, survival, and differentiation. Its role in these diverse processes is presumably carried out through interactions with other proteins, and there are at least 110 of these already identified. Despite our molecular understanding of how RB influences cell behavior, we still do not know precisely which of its functions endow RB with tumor suppressor activity.
At the outset of 2007, our research group at the Ohio State University, in collaboration with laboratories at Miami University and the University of Calgary, presented new data in a paper published in Genes & Development to suggest that the key to how Rb suppresses cancer may lie in its unique ability to regulate stem cell proliferation and possibly differentiation (Wenzel et al. 2007). The placenta is nearly fully formed halfway through gestation and arises rapidly after implantation into the uterus as trophoblast stem cells (stem cells of the placenta) differentiate into trophoblast cells of several lineages. These trophoblast cells provide the placenta with unique properties that are similar to those of most solid tumors, including aggressive growth, a strong blood supply, and resistance to attack by the immune system. We designed a transgenic mouse that made it possible to inactivate the Rb gene in trophoblast stem cells of the placenta while leaving Rb fully functional in all other tissues of the fetus and yolk sac (Fig. 1).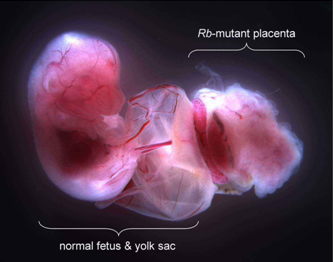
Figure 1. Mutation of the Rb gene was limited to trophoblast stem cells such that all cells of the fetus and yolk sac had a fully functional Rb gene and the placenta was mutant.
Mutation of the Rb gene in the trophoblast stem cells induced uncontrolled cellular proliferation in a portion of the placenta called the labyrinth, the primary site of maternal-fetal exchange. The abnormal growth was characterized by clumping and death of the placental trophoblasts and suggested a failure of the stem cells to properly differentiate and stop growing (Fig. 2). In fact, the Rb-mutant placenta was unable to support the life of the embryo, which was spontaneously aborted between 13 to 15 days of gestation. We were also able to determine that, in healthy placentas, Rb limits the activity of a protein called E2F3 that pushes cells to proliferate. By mutation of the gene that encodes E2F3 (E2f3), the Rb-mutant defects in the placenta were ameliorated and the life of the embryos was extended to a day before birth. In summary, these findings show that Rb keeps proliferation of trophoblast stem cells in check, at least in part, through the suppression of E2F3. The fact that Rb-E2f3 doubly-mutant embryos still do die, albeit days later, indicates that as of yet unknown functions of Rb are also likely critical for embryo development.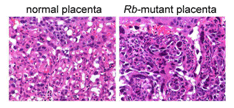 Figure 2. Loss of the Rb gene in trophoblast stem cells causes the normally spongy architecture (left photo) of the placental labyrinth to grow aberrantly (right photo). Clumping cells reduce blood space, cause localized death of placenta tissue, and compromise placental function.
Figure 2. Loss of the Rb gene in trophoblast stem cells causes the normally spongy architecture (left photo) of the placental labyrinth to grow aberrantly (right photo). Clumping cells reduce blood space, cause localized death of placenta tissue, and compromise placental function.
Implications for Human Pregnancies
Mutation of the Rb gene in mice is known to result in embryonic lethality approximately halfway through gestation (Clarke et al. 1992; Jacks et al. 1992; Lee et al. 1992). Our studies show that the cause of death is placental dysfunction (de Bruin et al. 2003; Wu et al. 2003; Wenzel et al. 2007). Since trophoblast cells of the placenta mediate metabolic conversion and all exchange between the fetal and maternal blood supply, any defect in these cells can adversely impact processing and transport of oxygen, hormones, nutrients, and waste, thereby increasing the likelihood of embryonic death and termination of the pregnancy.
Since Rb is critical for proper development of the mouse placenta, it is highly likely that RB is also important for the development of the human placenta. Those individuals who inherit a mutated RB gene from a parent are at risk for developing many types of cancers but, as studies in the mouse may suggest, could also be predisposed to suffer miscarriages or pre-eclampsia during pregnancy. As a vast majority of miscarriages are poorly understood and not linked to a specific gene defect, establishing the genetic basis of spontaneous abortion would be an important advance in medicine. This logic could also be extended to individuals who have not been affected by retinoblastoma but may have an underlying genetic predisposition for failed pregnancies. Ongoing research should begin to elucidate whether mutations in RB and other cell cycle regulatory genes may be responsible for placenta-associated complications during human pregnancy.
Implications for Stem Cell Biology and Cancer
One hypothesis in the area of cancer research is that cancer stem cells contribute to and propagate tumor growth (Reya et al. 2001). These are cells that, like normal stem cells, can self-renew and contribute to populations of differentiated, specialized cell types. Unlike normal stem cells, however, cancer stem cells give rise to large populations of highly proliferative cells and are oftentimes uniquely equipped to evade cancer therapies. Treatments that target and kill growing cells are sometimes ineffective because cancer stem cells have the capacity to rest in a relatively quiescent state. They are also found to express genes that aid in pumping chemotherapeutics out of the cell (Wulf et al. 2001, Zhou et al. 2001, Rossi et al. 2005), thereby detoxifying the internal cellular environment and endowing them with drug resistance. Coupled with an affinity for close association with the blood supply (Calabrese et al. 2007), these specialized and stem cell-like properties enable malignant cells to infiltrate tissues quietly and over-run the normal cell population.
To develop treatments that combat diseases that arise from uncontrolled proliferation, our attention turns to those genes that normally limit cell division by inducing cells to enter permanent quiescence and/or to terminally differentiate into specialized cell types. Rb plays a central role in the regulation of lineage specification, proliferation, and survival in a broad spectrum of tissues and cell types, including neurons, blood, fat, and muscle. We now know that it is also essential for the control of proliferation in trophoblast stem cells of the placenta. Together with the knowledge that the Rb pathway is consistently disabled in most human cancers, the role that Rb plays in the trophoblast stem cell makes it an attractive candidate for further study.
Stem cells of the placenta are particularly vulnerable to loss of Rb, possibly because genes that have similar or somewhat redundant functions do not have the capacity to functionally compensate in the stem cell environment. It is well known that two related genes, p107 and p130, frequently compensate for loss of Rb when the Rb gene is mutated in mouse models. Yet, unlike Rb, p107 and p130 are rarely found to be inactivated in human cancers and do not seem to be tumor suppressors. According to unpublished data from our research group, we have very little evidence to suggest that p107 or p130 play a significant role in trophoblast stem cells. These observations raise the possibility that stem cell-specific functions of Rb may be related to its unique ability to function as a tumor suppressor. In fact, it is well known that the interaction with E2F3 is unique to Rb and that p107 and p130, by and large, do not associate with this Rb-specific binding partner. By extension, it could be suggested that the suppression of E2F3 by Rb in trophoblast stem cells provides the first clue to identifying the unique property that endows Rb with tumor suppressor activity. Understanding how the Rb tumor suppressor gene regulates stem cell proliferation and renewal could provide unique insight into how the cell cycle is regulated in stem cell populations. This knowledge could represent a critical first step in understanding how Rb and other tumor suppressors function in cancer stem cells and may allow future therapies to target these cells in the process of cancer initiation and progression.
References:
Calabrese C, Poppleton H, Kocak M et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell. 2007; 69-82.
Clarke AR, Maandag ER, van Roon M et al. Requirement for a functional Rb-1 gene in murine development. Nature. 1992; 359: 328-330.
de Bruin A, Wu L, Saavedra HI et al. Rb function in extraembryonic lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proc Natl Acad Sci USA. 2003; 100: 6546-6551.
Jacks T, Fazeli A, Schmitt EM et al. Effects of an Rb mutation in the mouse. Nature. 1992; 359: 295-300.
Lee EY, Chang CY, Hu N et al. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992; 359: 288-294.
Reya T, Morrison SJ, Clarke MF et al. Stem cells, cancer, and cancer stem cells. Nature. 2001; 414: 105-111.
Rossi DJ, Bryder D, Zahn JM et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. PNAS. 2005; 102: 9194-9199.
Vogel F. Genetics of Retinoblastoma. Human Genetics. 1979; 52: 1-54.
Wenzel PL, Wu L, de Bruin A et al. Rb is Critical in a Mammalian Tissue Stem Cell Population. Genes Dev. 2007; 21: 85-97.
Wu L, de Bruin A, Saavedra HI et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature. 2003; 421: 942-947.
Wulf GG, Wang RY, Kuehnle I et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001; 98: 1166-1173.
Zhou S, Schuetz JD, Bunting KD et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nature Medicine. 2001; 7: 1028-1034.







I accept the article in it's current form. It is certainly a leap to extrapolate from a mouse developmental model to human cancer stem cells. However, your hypothesis that targeting the Rb pathway might have therapeutic significance for eradicating the presumably treatment-refractory cancer stem cells is notionally testable. I look forward to your further work in this area.
Great paper. I am not an expert on RB gene. This short review and other papers from the authors have enhanced my understanding of the RB gene and it role in stem cell biology and cancer.