Key words :
nanoscience,
supercurrent
,graphene
,ballistic transport
,dirac
,electronic properties
,transistor
,bipolar
A Theorist's Pencil and One Layer of Carbon Atoms, Graphene
26 Nov, 2007 02:45 pm
Graphite consists of layers of atoms arranged in a honeycomb lattice. A single layer of carbon atoms, called graphene has unique electronic properties. Graphene can be used to make a bipolar transistor and is a model system for relativistic two state quantum systems. Although macroscopic graphite is known and has been used for some time, only in the last few years measurements have been carried out on individual atomic layers.
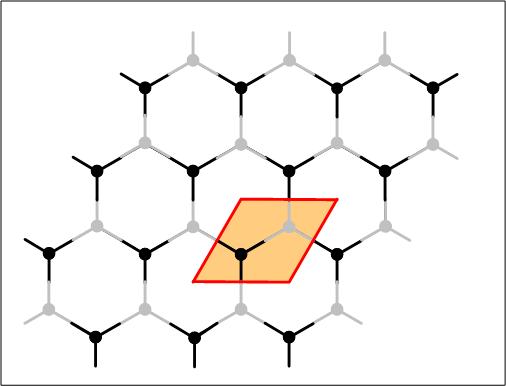 Graphene: Carbon atoms can form a planar honeycomb lattice. When stacking a bunch of them together they form graphite. The chemical bonds in the plane are extremely strong. The bonds between the planes are extremely weak. As a result, the planes of carbon atoms can slide on top of each other. Each time a pencil is used, part of the graphite is transferred to the paper; the shear force moves several graphene layers to the paper through the weak interlayer coupling. They are not single graphene sheets because single graphene sheets are too thin to reflect light significantly, they are in fact transparent.
Graphene: Carbon atoms can form a planar honeycomb lattice. When stacking a bunch of them together they form graphite. The chemical bonds in the plane are extremely strong. The bonds between the planes are extremely weak. As a result, the planes of carbon atoms can slide on top of each other. Each time a pencil is used, part of the graphite is transferred to the paper; the shear force moves several graphene layers to the paper through the weak interlayer coupling. They are not single graphene sheets because single graphene sheets are too thin to reflect light significantly, they are in fact transparent. A two dimensional metal: Carbon nanotubes are rolled up graphene sheets. They can be one nanometer wide and their electronic properties have been explored by attaching electrodes and by measuring the electronic transport properties. Highly ordered pylrolytic graphite (HOPG) is often used as a flat substrate or support for atomic force microscopy. A sheet of graphite can be peeled off by using adhesive tape. Scientists have in the last few years explored the electronic properties of a few or individual graphene layers. While carbon nanotubes can be semiconducting or metallic, graphene is metallic. Transistors are based on semiconductors where the electronic properties can be influenced by an applied electric field. This is not the case for metals. Due to the two dimensionality (1) of graphene however, it was found that the electronic transport does depend on an applied electric field. This makes it possible to use one layer of atoms attached to electrodes as a transistor. What is amazing is that a single graphene layer stays in tact and does not disintegrate the carbon bonds are strong enough to withstand interaction with the environment and strain within the layer. In fact when exploring the structure of graphite related materials using a high resolution electron microscope, one can witness a large variety of rather surprising three dimensional structures made out of graphene. Examples are the formation of graphene cones, ribbons or screws. The electronic transport property measurements on graphene show that at low temperatures, electrons can move ballistically, the current-voltage characteristics are linear, that graphene planes can sustain high current densities (about 100 times higher than in copper and about 10 times smaller than in carbon nanotubes) and shows high electron and hole motilities.
The theoristís pencil: Graphite is unique in that the full and empty energy bands touch at one particular point in reciprocal space. The electrons and holes in graphene follow in addition, a linear dispersion law which implies zero effective rest mass for the charge carriers. This has the result that the electrons are described by a relativistic wave equation in two dimensions (Dirac equation). The quantum field theorists who use pencils to write down equations find that the graphite which sticks on the paper is a highly unique model system to study the behavior of relativistic two dimensional quantum systems such as their conductance and non-localization.
How perfect? Graphene is highly crystalline and two dimensional, but theory and experiments show that two dimensional crystals are unstable and cannot exist. A latest work on the crystallographic perfection in a free standing layer of graphene shows that the graphene layer contains in fact, a roughness at the microscopic distance range and it is argued that this roughness is essential for the stability of graphene and may prevent the localization of charge carriers.
Supercurrents. When connecting a graphene layer to superconducting electrodes, a super current has been measured (Josephson effect) which can be explained by the formation of pairs of charge carriers (Cooper pairs) that completely changes their interaction. This means that a current is flowing without resistance. By changing the gate voltage, the charge conduction can be made to occur either through hole pairs or electron pairs enabling the device to be used as a bi polar supercurrent transistor.
Unexpected discovery: We can see how progress in microscopy and work on a similar system, carbon nanotubes has let to the discovery of physical properties in a single layer of atoms which differ from usual metallic or semiconducting properties. Graphene is a part of a very basic material we deal with on a daily basis and some call graphene one of the most versatile systems in condensed matter research (4). The history of science is full of such examples showing that important discoveries occur on unexpected occasions, a fact largely ignored by most funding agencies today.
Preferences:
1. K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva, A. A. Firsov, Science 306 (2004) 666
2. K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos, A. A. Firsov, Nature 438 (2005) 197
3. Jannik C. Meyer, A. K. Geim, M. I. Katsnelson, K. S. Novoselov, T. J. Booth & S. Roth, Nature 446 (2007) 60
4. A.H. Castro Neto, F. Guinea, N.M. Peres, A.K. Geim, cond-mat 0709.1163v1
Key words :







 Read more
Read more