Bio-inspired Super-strong Carbon Materials
2 Aug, 2007 02:08 pm
Our work in the July 26, 2007 issue of Nature demonstrates a materials platform that can be further tuned through chemistry to perhaps yield super-strong but lightweight structures with many applications. Structures in living systems offer us ?bio-inspiration?, and with that in hand, we can discuss how to take advantage of the superb intrinsic stiffness and strength of nanostructures such as a single layer of graphite, by configuring them in 3-dimensional space in the right way, and with the right molecular glue between them.
In order to understand the importance of this development, one must understand a few terms that have particular meanings to people who study the mechanics of materials. Strength and stiffness are often interchanged in our everyday speech, but in the field of mechanics they refer to different properties. A material might be very stiff, but not strong. Most ceramics are examples, where upon a small amount of mechanical load, they resist the load very well (they lengthen a very small amount). But with increasing mechanical load, they easily break; their strength is not as high as it could perhaps be. The energy that is expended to bring the sample from zero deformation all the way to fracture (breaking) while it is being mechanically loaded is called the fracture toughness. It would be useful to have materials that would be very stiff, very strong, and very tough, and even lightweight as well. An example of applications for such materials is in aerospace (among others). Let us now turn to a somewhat paradoxical material, comprised largely of the strongest bonds that can exist in a material but that is actually quite weak—it fractures fairly easily and is certainly not tough!
Graphite is composed of graphene sheets—all carbon layers that have each carbon bonded to 3 nearest neighbors in the “chickenwire” hexagonal arrangement. The carbon-to-carbon bond in graphite has been called the strongest bond that could exist in a material. (We acknowledge that the bond between the two nitrogen atoms in the molecule N2 is stronger—but it is a gas molecule, not a material.)
Why then, are pieces of graphite weak? Let us consider if there is a relationship between form and function for graphite! In architecture, we would hope that there is such a relationship, and in an ill-designed chair we uncomfortably realize that the designer has neglected the form-function relationship. Indeed, in our own bodies we benefit from an evolution of form and function. For example, in the structures of our bones and teeth, and in nature in general, there are many examples of stiff, strong, and tough materials. The abalone shell is one well studied example – a part of the abalone shell is composed primarily of inorganic platelets (calcium carbonate in the form called aragonite) with a relatively small amount of protein “glue” helping to hold the brickwork-style assembly together. The function of the shell is to protect the animal and the components, and most importantly, their configuration and interaction with each other, are the product of eons of evolution.
But graphite is not part of a living system. It is formed through the millennia by pressure and temperature beneath the surface of the earth, and in terms of strength, the platelets (the graphene sheets that are the individual layers) are not distributed nearly optimally. The crystallites in a piece of graphite have beautiful stacking in what is referred to as the c-axis direction (think of it like the z-axis), whereas the a-b plane (think of this like the x-y plane) is the hexaganol (some refer to this as “chicken wire” type bonding) network with the very strong C-C bonds. So, the crystallites have thousands of layers of graphene sheets, but the crystallites themselves are “butted up against each other” with little or no strong bonding between them. Please see the figure below which exaggerates this effect for emphasis, and shows how to better distribute the individual layers to perhaps make superstrong materials (that additionally can be very stiff, tough, and because graphite has low density, also lightweight).
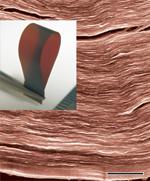
Figure 1:
A cross-section after fracture of a paper-material comprised of individual and layered sheets of graphene oxide (prepared by Sasha Stankovich). This is a scanning electron microscope image, scale bar is 1 micrometre in length. The inset shows a strip of this 'graphene oxide paper' held with metal tweezers. (Images by Dmitriy Dikin.)

Figure 2:
Top: A representation of 'crystallites' comprised of stacked layers, such as stacked graphene layers in graphite. Note that these layered stacks are not strongly connected with each other so that the aggregate would not well support a mechanical load.
Bottom: By disassembling these layers and then randomly assembling them in the same region, a molecular "glue" connecting the overlapping areas could form an aggregate with exceptional strength. This schematically shows Rod Ruoff's dream of "disassembling graphite to make new materials with the individual layers linked together with appropriate areal overlap between them, to yield superstrong materials that exploit the remarkable mechanical properties of the individual graphene sheets."
My concept has been that it might be a wonderful thing to disassemble graphite all into individual graphene sheets, and reassemble them in a way something like in the figure, which is intended as a conceptual guide. By then bonding the layers together “in the right way” the extraordinary mechanical properties of the individual graphene sheets might then be manifest in the resulting macroscopic samples that could be (by clever scientists) optimally configured from nanoscale through macroscale. Chemists need not use only C atoms in achieving super-strong materials composed almost entirely of graphene sheets…other elements are welcomed that allow optimizing the molecular glue that acts to allow optimal “mechanical load sharing.” The first step for me and my research team at Northwestern University has just been reported in the July 26, 2007 issue of Nature. The work as published is certainly not meant as “an end all” but rather as a very flexible materials platform that can be further optimized, particularly with respect to the mechanical properties but also with respect to electrical, thermal, chemical, and barrier properties. Indeed, it is a fun and stimulating “materials system” to study.
Would the abalone want to use graphene sheets, and would I want aragonite platelets in my zero carbon footprint, bio-fueled jet airplanes of the future? The answer is “no” to each question, in my opinion. The abalone constructs it shell from what is available in the surrounding environment and the function that results is what it needs in its environment. The aragonite platelet is, in tension, not nearly as intrinsically stiff or strong as the graphene platelet is, and so conversely our plane might not fly very well if the aragonite platelets were dominating its structure.
(Prof. Ruoff and his research group are relocating to the University of Texas at Austin in September, 2007. See: http://www.engr.utexas.edu/news/articles/200707251292/index.cfm)
Reference:
Dmitriy A. Dikin, et al, Preparation and characterization of graphene oxide paper, Nature 448, 457-460 (26 July 2007), p457






 Read more
Read more