Carbon Nanotubes as Deformation Cells on the Nanoscale
Nanocrystalline materials are composed of crystallites with sizes of a few nanometers. It has been observed since decades that such materials are mechanically very robust; their strength exceeds that of macroscopic crystals considerably. Thus, by decreasing the dimensions of the crystallites to the nanometer domain, the elastic and plastic behaviour of materials can change dramatically. Deformation experiments have already contributed to the understanding of the unusual mechanical properties of nanocrystalline matter, but the behaviour of individual nanometer-sized crystallites under stress has not been accessible to direct observation. Now, it has been found that carbon nanotubes, which are graphitic cylinders of ultimate tensile strength, can be used as deformation cells for single nanocrystallites [1]. The tubes can act as minuscule deformation tools, offering the ability to process materials as in a nanoscale jig or extruder.
The deformation of carbon nanotubes can be achieved when the tubes are subjected to intense electron irradiation.The experiments are carried out in an electron microscope where carbon atoms are knocked from the graphitic shells by the energetic electron beam. At the same time, the structure is imaged with atomic resolution. It has already been seen earlier that nanotubes shrink under electron irradiation although the tubes are not destroyed. This surprising observation has been explained by computer simulations. Vacancies in the graphite lattice (open sites which are left after the displacement of carbon atoms) have a high mobility at the temperature of the irradiation experiments (600°C) and coalesce to form divacancies. Divacancies are unstable, however, and collapse by closing open bonds so that a coherent graphite layer is restored. Such a layer does not just consist of hexagonal rings only as the perfect graphite lattice, but also of pentagonal or heptagonal rings. Hence, the graphite structure is able to heal itself when atoms are missing, and its high mechanical stability is preserved. However, the surface area is getting smaller so that cylindrically closed graphite shells contract under electron irradiation. 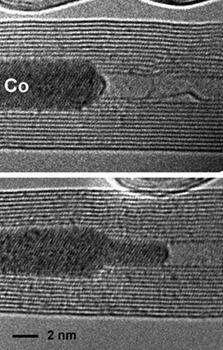
Figure:
A cobalt crystal is deformed inside a collapsing carbon nanotube under electron irradiation. Top: before irradiation; the Co crystal is still undeformed. Bottom: collapse of the nanotube leads to a thinning and deformation of the Co crystal.
If other materials are filled into the hollow nanotubes, a contraction of the tubes would act against the filling and eventually deform it. During the irradiation experiments, it is observed that the contraction of the tubes is so strong that the encapsulated crystals, e.g., metals or metal carbides, are massively deformed and finally squeezed through the tubes in the axial direction. Calculations show that pressures up to 40 GPa can be built up in the radial direction inside the collapsing tubes during such an experiment. This is by far enough to deform even hard materials.
This experiment enables us to study the deformation of individual nanometer-sized metal crystals. Carbon nanotubes serve as “nanolaboratories” for experiments with nanometer-sized crystals. The high resolution of the electron microscope where the in-situ experiment is carried out permits the observation of the deformation process with atomic resolution. The results also demonstrate the impressive strength of carbon nanotubes against internal pressure, which could make them ideal structures for nanoscale hydraulics and cylinders.
[1] L. Sun, F. Banhart, A. Krasheninnikov, J.A. Rodriguez-Manzo, M. Terrones, and P.M. Ajayan, Science 312, 1199 (2006)






