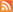Fossilized Liquid Assembly: A Bio-Inspired Route to Complex Structures
The advanced capabilities of many materials result from their exceptional surface properties. While coatings and paints improve surface properties through composition changes, some property improvements can also be achieved by changing the shape of the surface. One can cite many familiar examples: treaded tires for better traction, roughened windows for reducing transparency, and grooved records for reproducing sound. Notice that in each example, the choice of material is not critical. Windows can be made from glass or plastic, records can be made from wax or vinyl, etc. What makes this concept so powerful is the fact that dramatic improvements can be obtained from existing materials solely through the control of shape.
By Jason J. Benkoski*, Hua Hu, Alamgir Karim
The property enhancement can be even more dramatic when the surface features are shrunk down below microscopic length scales. Nowhere can this better be seen than in nature. The microscopic structure of shark skin has been known to play a crucial role in reducing frictional drag. The scales have fine grooves, about 0.1 mm wide, that run along the length of the shark. Relative to a smooth surface, the grooves make it easier for the shark to cut through the water. One may also look to the iridescent patterns on butterfly wings, which result from the way that light plays upon the multilayered, microscopic ridges on their surface. Such structures reflect light much more intensely compared to a colored surface, allowing the butterflies to see their mates from larger distances.
As seen above, different length scales are optimal for different applications. In the case of shark scales, the riblet size is determined by the spacing of tiny vortices that naturally form in water as it flows across a flat surface. Similarly, the size of the ridges on butterfly wings must be close to the wavelength of visible light. Other cases arise when tradeoffs between competing properties set the optimal length scale for the system. Even in these cases, nature has found a way to overcome this limitation. It creates a hierarchy of surface structures over multiple length scales. In this way, different properties can be independently optimized at different size scales of the hierarchy. Such hierarchically rough surfaces are responsible for both the amazing water repellency of lotus leaves and the unmatched adhesive properties of gecko feet. The gecko sticks to surfaces because its feet are patterned with microscopic hairs, each hair tipped with hundreds of even tinier projections. Beads of water roll off the lotus’s leaf because its surface is streaked with microscopic peaks, each with a finer structure, that makes the surface “super hydrophobic.” When properly applied, this strategy makes it possible to maximize the desired properties while avoiding any unwanted side effects. These enhanced properties have attracted the attention of design engineers for applications from bioengineered tissues to photoelectronic materials to submarines that slice through water with minimal drag.
The main lesson to be gained from these examples is that extraordinary properties can be achieved with ordinary materials. Observe that none of the materials used by nature are particularly exotic: keratin (gecko feet), wax (lotus leaves), cellulose (wood), hydroxyapatite (teeth), calcium carbonate (bone), and chitin (insect shells). In their unstructured form, they are a far cry from engineering materials such as Kevlar™, Teflon™, or nickel superalloys. However, we see that the choice of material appears to depend less on superior properties than on availability to the particular organism. In this respect, nature makes a strong case for focusing on advanced processing rather than advanced chemistry.
Creating these topologically complex, self-assembled surfaces for study has been a challenge. If the components are mixed on a surface, that surface affects how they assemble; if mixed in a solvent and dried, the drying process similarly distorts the results. In a recent paper*, we have detailed a much simpler and faster system dubbed “fossilized liquid assembly” to create experimental models of hierarchical topologies in which the components are allowed to mix and assemble freely in a fluid, and then quickly “frozen” in place for study. The key is the use of solutions of water and a special type of oil that polymerizes—solidifies—when exposed to ultraviolet light. Like shampoo or lotions or salad dressing, the fluids form liquid interfaces that can be manipulated to create complex structures. With our approach, these structures can then be suddenly solidified at the instant at which it is exposed to UV light. For example, our paper* demonstrates that by shaking (or vortexing) the oil-water interface with added nanoparticles, one obtains a structure in which large spheres are covered by smaller spheres, which, in turn, are covered by even smaller spheres. Such raspberry-like structures possess all of the essential features of the lotus leaf.
Figure : Optical microscope image (lower plane) shows spheres at mutiple size scales self-arranging in complex "super-assemblies" in NIST's hierarchical topology modeling system. Atomic-force microscopy (detail) shows the textured surface formed by the spheres.
Credit: NIST
Using this approach, a finished product can be prepared in 5 to 10 minutes. Not only are competing methods more costly and time-consuming, but they do not allow such complex self-assembled structures to form so freely. This new technique also makes it possible to build complex dynamic structures and freeze them into solid form, providing a quick and easy method for viewing them under a high-powered microscope. By solidifying the oil phase, it overcomes the limitations of imaging small, delicate, and highly mobile structures in a liquid medium. Providing a solution to these problems represents a major breakthrough in the study of self-assembly, since most microscopy techniques with sufficient magnifying power either suffer from slow scan rates or require measurements performed in vacuum. This new method is also gentler than previous sample preparation techniques, which means that the original shape of these delicate structures can be better preserved, or “fossilized”. Looking ahead, we plan to build on this study, expanding the technology as a 3D imaging platform.
* J.J. Benkoski, H. Hu, and A. Karim. Generation of hierarchical topologies from photocrosslinkable, particle-stabilized emulsions. Macromolecular Rapid Communications. Aug. 2, 2006