Nanotechnology for Healing Damaged Vascular Tissue
4 May, 2007 12:45 pm
Atherosclerotic vascular disease, characterized by artery hardening and vessel narrowing through plaque accumulation, has become the leading cause of death in western countries. It serves as one of the main factors contributing to coronary heart disease. Specifically, according to the American Heart Association, more than 39 million of all the deaths in the United States have been associated with cardiovascular disease (Heart Disease and Stroke Statistics 2004). In addition, the National Institutes of Health (NIH) in 2005 reported approximately 58 million deaths as a result of atherosclerotic vascular diseases.
Coronary artery bypass grafting is a surgery during which physicians create a detour around a narrow vessel blocked as a result of atherosclerosis using autologous vessels or synthetic vascular grafts to serve as replacements of the diseased natural vessels. Vascular stenting is the procedure of implanting a thin metal (such as Ti, stainless steel, Nitinol or CoCr alloys) tube into the part of the artery blocked by plaque accumulation in order to prop it open and reestablish blood flow.
However, numerous problems exist with each of these two treatments, all centered on minimizing “body rejection” or the inflammatory response that follows any biomaterial insertion. Conventional synthetic bypass graft materials and metallic vascular stents as formulated today are generally not biocompatible with tissues; this clearly affects the behaviors of the cells that are found in healthy arteries (such as the vascular endothelial cells and smooth muscle cells). As a result, implantation processes using conventional (or nano-smooth) materials induce excessive vascular smooth muscle cell function causing significant further injury to the vascular wall and endothelium. The endothelium, which consists of a layer of endothelial cells, functions as a protective biocompatible barrier between the vascular tissue and circulating blood. Its injury could result in neointima hyperplasia and then the development of long-term restenosis, leading to artery and bypass re-blockage by scar tissue. Re-blockage of arteries usually occurs in more than 25% of patients within 3-6 months post-operation.
Nanotechnology Solutions to Improve Vascular Implant Performance:
Therefore, to reduce postoperative failures, the inhibition of vascular smooth muscle cell functions and the rapid repair of an endothelium are critical. Numerous studies have focused on these issues to improve conventional synthetic bypass grafts and vascular stent materials. For example, for bypass grafts, thinking beyond traditional materials like Dacron, polyurethane, and polytetrafluoroethylene (PTFE), a novel class of polymers such as poly(lactic acid), poly(glycolic acid), and their co-polymers (PLGA) have been developed, while for vascular stents, until as of late drug containing polymer coatings on bare metallic stents have been widely used. However, all of these improvements are based on micron-scale technology and not nanotechnology. Only nanotechnology can mimic the natural nano-structure of our tissues, especially arteries which possess a unique nano-scale texture and roughness.
In contrast to most current efforts, the researchers in the lab of Professor Thomas J. Webster (Nanomedicine Laboratories, Brown University, Providence, RI) have focused on designing better synthetic vascular polymeric grafts and metallic vascular stents using novel nanotechnology approaches. They have demonstrated for the first time that when compared with implants which are nano-smooth, those with nano-scale surface features mimic the natural vessel walls to attract more endothelial cells and promote their growth, thus, accelerating the re-formation of an endothelium to prohibit re-blocking of the artery. Thus, they have shown that nanosurfaces on metallic vascular stents (Figure 1) and polymeric vascular grafts (Figure 2) might “save” the implant from suffering a detrimental inflammatory response. The following experiments confirmed this hypothesis.
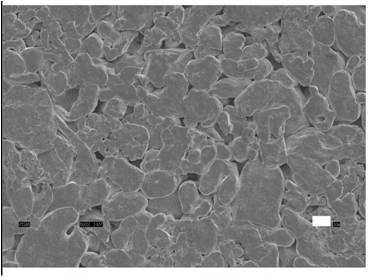
Figure 1. Surface of a Nanostructured Titanium Vascular Stent Composed of Titanium Nanoparticles. Scale Bar = 1 mm.
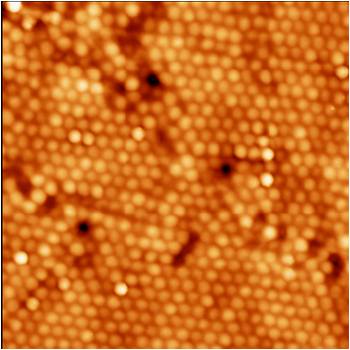
Figure 2. Surface of a Vascular Graft Composed of Polymer (Poly-lactic-Co-Glycolic-Acid or PLGA) Nanospheres. Scale Bar = 200 nm.
One successful experiment on metallic vascular stent materials was accomplished by Saba Choudhary, Professor Thomas J. Webster and Professor Karen M. Haberstroh. They created nano-scale surface features by pressing together pure titanium particles with diameters of several hundred nanometers (Figure 1). Then, vascular endothelial and smooth muscle cells were seeded separately on those substrates as well as conventional (or nano smooth) titanium. After 4 hours, more endothelial cells adhered compared to that observed for vascular smooth muscle cells. After 5 days, an intact monolayer of endothelial cells (an endothelium) was formed on the nano-scale surface features before smooth muscle cells formed a layer. These in-vitro results suggested that on the vascular stents with nanosurfaces, endothelium repair would be completed before those stents with conventional topographies.
But metals are not alone in this trend. Promising results have also been found for vascular bypass polymer graft research. Here, Derick Miller, Webster and Haberstroh succeeded in casting PLGA (poly-lactic-co-glycolic acid, a common FDA approved biodegradable polymer) on polystyrene nanospheres (100nm, 200nm and 500 nm in diameter: Figure 2) to create novel nanostructured polymer vascular grafts. Similar to nanostructured metals, vascular endothelial cell adhesion experiments showed greater endothelial cell density on nano-rough PLGA made from the mold of the 200 nm nanospheres than the nano-smooth surfaces.
While much more work must be conducting, including animal testing, these results collectively show great potential for the use of nanomaterials (metals and polymers) in treating various cardiovascular diseases which require a quick re-formation of an endothelium (such as in arteriosclerosis where the endothelium has been disrupted).
Reference:
Derick C. Miller et al., Journal of Biomedical Materials Research, Part A, 81A, Issue 3, 678-684







Sounds intersting and appears technically correct