Slippery Nanopipes
15 Jun, 2006 10:32 am
Imagine the amount of water that comes out of a fire hose attached to a hydrant. If you replace the fire hose with a regular garden hose, you expect to get considerably less water from the hydrant in a given amount of time. In the macroscopic world familiar to us, we do not have special hoses that are thin but can deliver as much water as the fire hoses. We have recently discovered that carbon nanotubes, nanoscale hollow cylinders 50,000 times thinner than a human hair, are capable of performing such a trick. Despite their small inner diameter, carbon nanotubes allow a relatively fast flow of water and gas through them. We reported this research in the May 19th 2006 issue of Science.
Filtration is an obvious practical application of permeable pores whose size is comparable to the size of molecules. Membranes that have carbon nanotubes as pores could be used in desalination and demineralization, gas separations, and even separations of biomolecules in devices that act as artificial kidneys. Extremely permeable filters would require less energy for their operation, which is important because energy is the major cost for applications such as desalination. Salt removal from water, commonly performed through reverse-osmosis, uses less permeable membranes, requires large amounts of pressure, and as a result, is still quite expensive. More permeable nanotube membranes could reduce the energy costs of desalination. Another potential application for the membranes is gas separation. The high gas permeability and its affinity for hydrocarbons may enable more efficient industrial gas separations. We may be able to use carbon nanotube membranes for cost-effective removal of greenhouse gases from power plant flue gas, which would have a profound effect on the environment. Even though the practical applications of carbon nanotube membranes may be years away, we are optimistic that the unique nanoscale phenomenon that we have observed in our experiments will lead to improvements in molecular separation technology.
A number of molecular dynamics simulations concerning the behavior of water molecules in very small nanotubes have been described in the literature. We wanted to see what would happen in an experiment. We fabricated a membrane with carbon nanotubes as transverse pores. The membrane is supported on a silicon chip the size of a quarter (Figure 1). By filling the gaps between the carbon nanotubes, we have ensured that molecules traversing the membrane must move through the internal lumen of the nanotubes. Before performing the measurements described in the Science paper, we were skeptical that water would enter carbon nanotubes, let alone flow extremely fast, since it has been our experience that water does not wet nanotube outside surfaces.
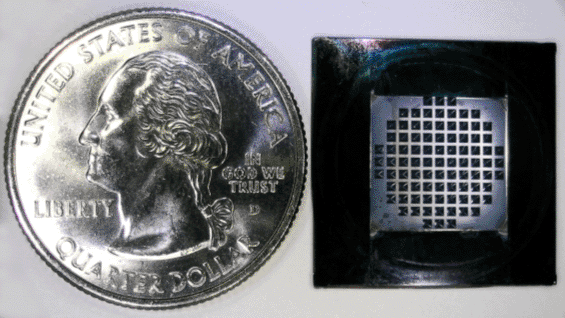
Figure 1: Sub-2nm carbon nanotube membrane chip.
The first time we set up an experiment, we filled the top of the membrane with water and left it overnight, thinking that the water level would not budge. To our surprise, when we came back in the morning, there was a little water puddle on the floor underneath the membrane. The first thing that came to mind was that the membrane broke, but later tests proved otherwise. This very same membrane that allowed water through was able to block small gold nanoparticles that were just a bit larger than the nanotube pores. Water was able to move from one side of the filter to the other, through the lumen of the nanotubes spanning the thickness of the membrane.
In these experiments, we have not only confirmed that the water molecules flow through our membrane, but have also shown that the flow of gas and water is respectively 100 to 10,000 times faster than what classical models predict.
Carbon nanotubes are a unique platform for studying molecular transport and nanofluidics. We believe that understanding how molecules behave as they move through our carbon nanotube filter may also help us understand the biology and physiology of how water molecules and ions are shuffled through vital protein channels in cellular membranes. We are working to more fully understand the molecular mechanisms and physical forces acting at the nanoscale that are responsible for the fast transport. Small molecules may travel fast through the carbon nanotubes, in part, because carbon nanotubes have slippery walls. Gas and water molecules could be bouncing off the nanotube’s atomically smooth luminal surface like billiard balls.
For more information contact: Olgica Bakajin
Figure 2: Artist's vision of methane molecules travelling through a carbon nanotube. Image by Scott Dougherty.






