Targeting and Detecting Cancer Cells Using Nanoparticles
23 Jul, 2007 02:26 pm
Current cancer therapy always experiences non-specific killing of healthy cells, thus the therapeutic efficiency is extremely low. Development of a nanoparticle system that can specifically target, detect, and kill cancer cells remains a great challenge.
The advances in nanoscience and nanotechnology greatly promote the rapid development of various new systems for cancer therapy in current medicine. An ideal nanoparticle platform should be endowed with targeting, imaging, and therapeutic functionalities, which allow for targeting, imaging, and killing of cancer cells. Dendrimers are a new class of highly branched, narrowly dispersed, synthetic macromolecules with defined composition varying from generation to generation (Tomalia, Naylor et al. 1990). The unique structural properties of dendrimers make them ideal candidates for use as a multifunctional platform to target, detect, and kill cancer cells (Kukowska-Latallo, Candido et al. 2005). In order to clearly understand the molecular mechanism of targeted drug delivery using multifunctional dendrimer nanodevices as well as to combine chemical therapy with physical therapy, we set out to design a multifunctional nanoplatform based on dendrimer-entrapped metal nanoparticles.
Dendrimer-entrapped metal nanoparticles have been primarily used in catalysis, optics, and other applications unrelated to biomedical sciences. The absence of biological applications is largely due to the toxicity of these particles and technical difficulties with their surface manipulation. We demonstrated that dendrimer-entrapped gold nanoparticles (Au DENPs) prepared using amine-terminated generation 5 poly(amidoamine) dendrimers as templates can be surface modified with neutral or close to neutral charged groups, thereby significantly reducing the toxicity of the particles (Shi, Wang et al. 2007). The ability to chemically functionalize preformed Au DENPs without significantly changing their sizes and size distributions led us to develop these particles as a multifunctional platform for cancer cell targeting, imaging, and treatment. One major advantage of using these hybrid dendrimer/gold nanoparticles to image cancers is its ability to differentiate cancer cells from surrounding cells or tissues by using contrast agents with high electron density, which allows imaging the nanoparticle distribution in subcellular compartments of cells using transmission electron microscopy. This can not be realized using dendrimer-based therapeutics because of the low electron density contrast. The use of Au DENPs also aids in understanding the mechanism for targeted drug delivery and therapeutics, using dendrimer-based nanodevices. In addition, it is possible to use the dendrimer/gold hybrid nanoparticles for laser thermo therapy of cancer through inductive heating of cancer cells that specifically internalize the particles.
We demonstrate that Au DENPs can be covalently linked with targeting and imaging molecules for cancer cell targeting and imaging (Shi, Wang et al. 2007). The vitamin folic acid has been extensively investigated for targeting various cancer cells, including ovary, kidney, uterus, testis, brain, colon, lung, and myelocytic blood that overexpress folic acid receptors. Au DENPs (the diameter gold nanoparticles is 3.2 nm) linked with 4-5 folic acid and dye fluorescein molecules are water-soluble, biocompatible and stable at a pH ranging from 5.3 to 8.4. Our molecular dynamics simulation studies show that the attached dye molecules on the Au DENP surfaces are distant from the gold nanoparticles, which minimize the fluorescence quenching effect with metal gold, while the attached folic acid molecules extend substantially outward, thus becoming available for interaction with folic acid receptors on the surface of target cells (Figure 1). These properties allow the hybrid dendrimer/gold nanoparticles to perform perfectly in the binding and detection of cancer cells through receptor-ligand interactions. We show that the folic acid and fluorescein-modified Au DENPs can specifically bind to KB cells (a human epithelial carcinoma cell line) that overexpress high affinity folic acid receptors and be internalized predominantly into lysosomes (cell organelles that contain digestive enzymes) of target cells within 2 h. These findings document a facile approach to use Au DENPs as a platform for the targeting and imaging of cancer cells.
It should be noted that the developed hybrid nanoparticle system can be extended to other targeting system (e.g., proteins, antibodies, peptides, sugars, etc.) for targeting and imaging of various biological systems. In addition, cancer drugs could also be conjugated onto the surface of Au DENPs through the well-established conjugation chemistry, thereby providing a truly unique multifunctional nanoplatform that can combine both physical and chemical therapy of cancer. We are currently conducting in vivo experiments to evaluate the suitability of this system for clinical applications.
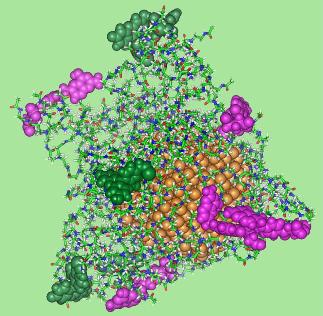 Figure 1. A selected equilibrated configurations of {(Au0)51.2-G5-FI5-FA5-Ac} DENPs. Atoms in the poly(amidoamine) dendrimer platform are shown as sticks, gold atoms as gold spheres, atoms comprising folic acid as pink spheres, and atoms for fluorescein as green spheres.
Figure 1. A selected equilibrated configurations of {(Au0)51.2-G5-FI5-FA5-Ac} DENPs. Atoms in the poly(amidoamine) dendrimer platform are shown as sticks, gold atoms as gold spheres, atoms comprising folic acid as pink spheres, and atoms for fluorescein as green spheres.References:
Kukowska-Latallo, J. F., K. A. Candido, et al. (2005). "Nanoparticle Targeting of Anticancer Drug Improves Therapeutic Response in Animal Model of Human Epithelial Cancer." Cancer Res. 65: 5317-5324.
Shi, X., S. Wang, et al. (2007). "Dendrimer-Entrapped Gold Nanoparticles as a Platform for Cancer-Cell Targeting and Imaging." Small 3: 1245-1252.
Shi, X., S. Wang, et al. (2007). "Improved Biocompatibility of Surface Functionalized Dendrimer-Entrapped Gold Nanoparticles." Soft Matter 3: 71-74.
Tomalia, D. A., A. M. Naylor, et al. (1990). "Starburst dendrimers: control of size, shape, surface chemistry, topology and flexibility in the conversion of atoms to macroscopic materials." Angew. Chem. Int. Ed. Engl. 29: 138.







 Read more
Read more
A very interesting report on utilizing dendrimer/gold hybrid nanoparticles as a multifuntional platform for the therapy of cancer.