The Adult Brain Can Rapidly Reorganise to Compensate for Functional Interference
21 May, 2007 12:40 pm
When a person suffers brain damage, for example a stroke, their ability to move their limbs is often impaired. Over time, some degree of recovery can occur naturally. The brain mechanisms enabling this recovery are not yet understood.
The picture from patient studies is complex. Some evidence suggests the new activity is adaptive [1,2]. On the one hand, increased activity is especially prominent in patients who show poor recovery [3]. To investigate this issue, we studied the brains of healthy volunteers. This avoided the complications of studying patients, who all differ in the location and extent of brain damage, as well as in symptom severity and in the degree and speed of recovery.
We used a method called transcranial magnetic stimulation (TMS), in which magnetic pulses are applied to the scalp [4,5]. They pass painlessly through the skull and induce electrical current in the brain. The induced current perturbs the normal pattern of activity in the targeted brain region. By analogy: when you listen to the radio, there’s often crackling on the line. You can still hear the presenter, only less well. We’re doing something similar - temporarily adding noise to the information passing through that area. The brain area continues to function, but less efficiently.
We used TMS to temporarily disrupt normal activity in a brain area called the dorsal premotor cortex, thus simulating brain damage to that area. We then gave people a task. Shapes appeared on a computer screen, and people had to press a button with one of two fingers, depending on which shape they saw. To quickly select the correct finger response, you need normal levels of activity in the dorsal premotor cortex.
As expected, after TMS, people were slower at the task. However, they recovered quickly – within 4 minutes. This suggested to us that the brain might have rapidly reorganised itself to compensate for the interference.
We imaged people’s brains and found that was exactly what had happened. During recovered performance, just like in stroke patients, there was increased activity in the ‘healthy’ half of the brain. In particular, the dorsal premotor cortex in the other hemisphere became more active. Importantly, the new activity was functionally specific. It emerged only during periods when people were performing the function – response selection – that is normally carried out by the brain area we had disrupted.
We performed one final crucial test. After the brain had reorganised itself, we then disrupted the reorganised activity. Sure enough, performance on the task was once again impaired. This made it clear that the function of the disrupted brain area had been ‘moved’ to the other half of the brain.
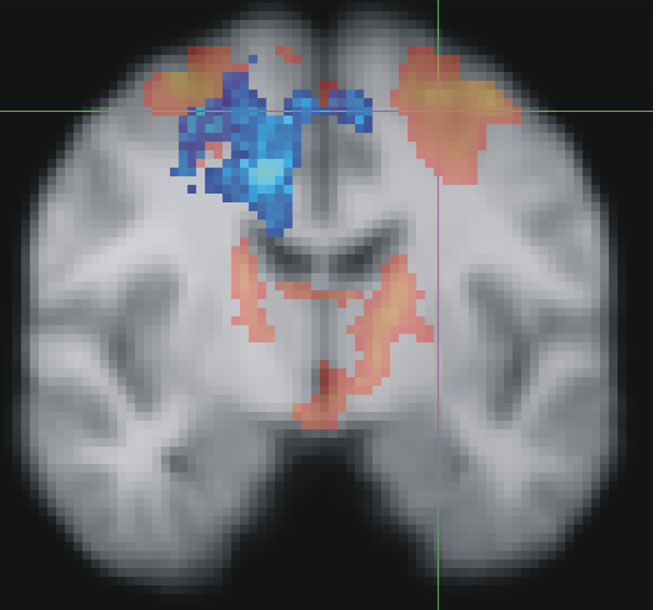
Figure :
Red shows the brain areas normally activated when people select the correct finger movement. Cross-hairs show where TMS was applied to disrupt activity in the dorsal premotor cortex. Blue shows how the brain recovered from the simulated brain damage: by transferring function from the ‘damaged’ region over to the ‘undamaged’ side of the brain. When the reorganised activity (blue) was disrupted, recovered performance broke down.
Our results establish three clear findings about the brain’s capacity to functionally reorganise: 1) it can occur with impressive speed; 2) it follows a precise pattern – connected brain areas that perform similar functions are the first to ‘take over’ from the compromised area; 3) the reorganisation is adaptive and can causally mediate recovery.
There is still a gap between these observations and the ultimate goal: to design successful treatment interventions for stroke victims. Nevertheless, our study has clearly identified one precise pattern by which the brain reorganises itself to overcome the effects of ‘injury’.
The more we can understand about the brain’s capacity to ‘heal itself’, the greater the potential for future clinical interventions to manipulate those mechanisms to enhance stroke recovery. Mutually informed studies from the laboratory and the clinic will speed progress towards that goal.
*Research funded by the Medical Research Council, U.K. JO’S is supported by the Stevenson Junior Research Fellowship from University College Oxford.
References:
1. Johansen-Berg, H., Rushworth, M.F.S., Bogdanovic, M.D., Kischka, U., Wimalaratna, S., and Matthews, P.M. (2002). The role of ipsilateral premotor cortex in hand movement after stroke. Proceedings of the National Academy of Science USA 99, 14518-14523.
2. Lotze, M., Markert, J., Sauseng, P., Hoppe, J., Plewnia, C., and Gerloff, C. (2006). The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. Journal of Neuroscience 26, 6096-6102.
3. Ward, N.S., Newton, J.M., Swayne, O.B., Lee, L., Thompson, A.J., Greenwood, R.J., Rothwell, J.C., and Frackowiak, R.S. (2006). Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 129, 809-819.
4. O'Shea, J., and Walsh, V. (2007). Transcranial magnetic stimulation. Current Biology 17, R196-199.
5.O'Shea J, Johansen-Berg H, Trief D, Gobel SM, Rushworth MFS (2007), Functionally-Specific Reorganisation in Human Premotor Cortex. Neuron 54: 479-490







The author did a good job to summarize the recent article in Neuron by O'Shea and colleagues and to make these findings clear to the average reader. This article stimulated my interest to find the Neuron paper, which, I think, is one of the goals of Scitizen.
The version of the article that I received via email had a number of internet HTML codes within the text, which need to be corrected before publication.